Volunteer for Research
Volunteer for Research

What to Know Before Participating in a Northwest Mental Illness Research, Education, and Clinical Center (NW MIRECC) Research Study
The U.S. Department of Veterans Affairs (VA) ranks as one of the nation's leaders in health research. Every year, thousands of research studies are conducted at VA medical centers, outpatient clinics, and nursing homes. NW MIRECC conducts research that seeks new ways to diagnose, treat, and prevent the following conditions in Veterans;
- Post-Traumatic Stress Disorder (PTSD)
- Mild Traumatic Brain Injury (mTBI), which is also called a concussion
- Chronic pain (post-concussive headaches) or other conditions that might start after an mTBI
- Gulf War Illness
- Drug abuse and dependence
- Dementia and neurodegeneration, including mild cognitive impairment (MCI) and Alzheimer’s disease (AD)
- Pandemic stress
Research studies may feature the following kinds of activities;
- Questionnaires, interviews, or assessments of physical and mental health
- Blood draws
- Talk therapies, which may include psychotherapy or cognitive rehabilitation interventions
- Tests that measure eye responses to light
- Lumbar punctures, which are used to obtain cerebrospinal fluid (CSF) and which have been safely performed in the NW MIRECC for more than two decades
- Scans of the brain, including diffusion tensor imaging (DTI), magnetic resonance imaging (MRI, fcMRI, or fMRI), and positron emission tomography (PET or FDG-PET)
- Prescriptions for prazosin, a medication that may be useful for a number of conditions
- Wearing wristwatches or other devices that track physical activity or sleep patterns
VA Research Contributions
These research studies have significantly contributed to advancements in our understanding of medical problems and that have led to health improvements for Veterans and civilians alike. For example, the VA has:
- Developed artificial limbs that allow amputees to have more independence and better quality of life
- Invented the cardiac pacemaker
- Performed the first successful liver transplantation
- Developed the nicotine patch to help people stop smoking
- Played a major role in the development of the CT/CAT scan to view the inside of the body
- Tested new drugs and treatments for diseases such as AIDS, Diabetes, Alzheimer's and Osteoporosis
 None of the advances in health care would be possible without individuals like you volunteering to participate in research studies.
None of the advances in health care would be possible without individuals like you volunteering to participate in research studies.
What is a research study?
A research study is an organized activity to learn more about a problem or answer questions. For example, a study may test if a product, such as a drug or equipment, is safe and effective. A study may be done to find out what health care practices work best. A study may be done to determine the best way to treat an illness or how to prevent an illness.
A study may use a survey or an interview to understand health needs, problems, or feelings people have about an illness or their general health. Another type of research study is a clinical trial. A clinical trial is a medical study with people that will try to determine whether medicines, new therapies or new devices are safe and effective. In clinical trials, drugs or treatments are often compared with placebos to check the effectiveness of that drug or treatment. A placebo is an inactive substance which may resemble an active substance. However, it typically has no value to treat or prevent an illness.
Why volunteer to participate in a research study?
There are many reasons to volunteer to participate in a research study. They include:
- to help find a cure for an illness
- to help other people who are sick
- to help find ways to provide better care
- to help scientists find out more about how the human body and mind work
- to take part in a study that is trying to find a better treatment for a condition that you have
If you decide to take part in a research study, you do so as a volunteer. That means YOU decide whether or not you will take part. If you choose to do so, you have many important rights.
Are there risks or side effects in a research study?
 Sometimes research procedures and drugs may cause discomfort and/or side effects. The questions being asked could make you uncomfortable. The risks and side effects of the research may not be known completely when you start the study. The research staff will discuss with you known possible risks so you can decide if you want to volunteer. If you do volunteer, the research staff will tell you about any new risks that they learn about during the study for as long as you participate in the study.
Sometimes research procedures and drugs may cause discomfort and/or side effects. The questions being asked could make you uncomfortable. The risks and side effects of the research may not be known completely when you start the study. The research staff will discuss with you known possible risks so you can decide if you want to volunteer. If you do volunteer, the research staff will tell you about any new risks that they learn about during the study for as long as you participate in the study.
Before you decide to volunteer to take part in a research study, you need to know as much as possible about the research study. If there are any issues that concern you, be sure to ask questions. You might want to write your questions down in advance. The following are a list of questions. Not every question will apply to every study.
- Will I be told the results of the study?
- How do I end my participating in this study if I change my mind?
- Who will find out that I am taking part in this study?
- Whom do I contact for questions and information about this study?
- What will happen to any specimens that I give?
- Who is doing this study and what questions might it answer?
- Will this research help in understanding my condition? If so, how?
- What tests or procedures will be done?
- Will I have to make extra trips to the VA?
- What could happen to me, good and bad, if I take part in the study?
- How long will this study last?
- Who has reviewed and approved this study?
- Could my condition get worse during the study? What will happen if it does?
- Is it possible that I will receive a placebo (inactive substance)?
- What other options or choices do I have if I decide not to take part in this study?
- Who will be in charge or my care?
- Will I be able to continue to see my own doctor?
- Will I be charged anything or paid anything to be in this study?
- If I decide to participate in this study, how will it affect my daily life?
- What will happen to me at the end of the study?
If you do not understand the answer to one of your questions, ask the question again and ask the person to explain the answer in a way you can understand. If you forget the answers to the questions during the study, just ask them again.
Are there benefits to being in a research study?
There may or may not be a direct benefit to you if you take part in a research study. For example, your health or a health condition you have may get beter as a result of your participation in the study, it may stay the same, or it may even get worse. No one can completely predict the outcome of a research study or how it might affect you. The study may not help you personally, but your participation in the study may result in information that will help others in the future.
What is informed consent?
 Informed consent is the process of learning the key facts about a research study before you decide whether or not to volunteer. Your agreement to volunteer should be based upon a clear understanding of what will take place in the study and how it might affect you.
Informed consent is the process of learning the key facts about a research study before you decide whether or not to volunteer. Your agreement to volunteer should be based upon a clear understanding of what will take place in the study and how it might affect you.
Informed consent begins when the research staff explains the facts to you about the research study. These facts include details about the study, tests or procedures you may receive, the benefits and risks that could result, and your rights as a research volunteer. The research staff will assist you with the "informed consent form" so you can decide whether or not you want to take part in the study.
You should take your time when you read the consent form. If you have any questions, ask the research staff. If you do not understand something, ask them to explain it in a way that would be more meaningful to you. If English is not your first language, you can ask for an interpreter to be present when you are discussing the study with the research staff. The written and verbal informed consent information will be given to you in a language that you know. You can take the information home with you and discuss it with your family, friends, health care provider, or others before you decide whether or not to take part in the study.
If you decide to take part in the study, you will be asked to sign the informed consent form. However, the informed consent process is more than just signing a piece of paper. It is a process that goes on throughout the study. During the course of the study, you may be told of new findings, benefits or risks. At that time, you can decide whether or not to continue your participation in the study. You may change your mind and leave the study before it starts or leave at any time during the study or the follow-up period.
Who will see my records?
Like your medical record, the information in your research record will be confidential. Information will be given only to the researchers who carry out the study or to those who make sure that the study is safe and carried out the way it was planned. The groups of individuals who might look at your records are the research staff, the Institutional Review Board (IRB), the company or group funding the study, and various government oversight agencies. It is important for these groups to be able to look at your records so they can ensure that the study is conducted using acceptable research practices.
About the Institutional Review Board or IRB
The Institutional Review Board (IRB) is a group of people such as doctors, nurses, pharmacists, scientists, ethicists, and people from local community who ensure that human research is well-planned and ethical.
The IRB of this medical center serves to protect your rights and your welfare before and during the research study. For example, the IRB makes sure that any risks in the research study are as small as possible. The IRB does not make a decision for you. The IRB decides, when approving research studies, that it is reasonable to ask people whether they want to be involved in it. The IRB also reviews each study while it is going on to make sure volunteers are protected.
In the VA, there is another committee called the Research and Development (R&D) Committee. This committee reviews the work and recommendations of the IRB and must also approve the research before you can be asked to take part in a study. This the VA’s way of assuring YOU that any study you are asked to participate in has been thoroughly reviewed.
What if I do not want to participate in a research study?
 Your participation in a research study is a strictly voluntary. You have the right to say “no.” Your decision to not participate will not affect your VA health care or other benefits.
Your participation in a research study is a strictly voluntary. You have the right to say “no.” Your decision to not participate will not affect your VA health care or other benefits.
Before you decide to participate, remember:
- Weigh both the risks and benefits of the research study
- It may be helpful to speak with your family members, friends, or health care providers
- If you decide to volunteer for a research study, you can change your mind and stop or leave the study at any time without losing any of your VA health care or other benefits.
For information about specific research, please contact the number listed on the research study.
NW MIRECC disseminates mission-relevant knowledge through publications and continuing health care education programs across VISN 20 and nationally. Sign up for email updates or access your subscriber preferences: https://public.govdelivery.com/accounts/USVHAVISN20/subscriber/new.
Use of these Materials and Finding VA Health Care
Please note that the health care information provided in these materials is for educational purposes only. It does not replace the role of a medical practitioner for advice on care and treatment. If you are looking for professional medical care, find your local VA healthcare center by using the VA Facilities Locator & Directory. This page may contain links that will take you outside of the Department of Veterans Affairs website. VA does not endorse and is not responsible for the content of the linked websites.
VA Web Disclaimers
Disclaimer of Endorsement: Reference herein to any specific commercial products, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government, and shall not be used for advertising or product endorsement purposes.
Disclaimer of Hyperlinks: The appearance of external hyperlinks does not constitute endorsement by the Department of Veterans Affairs of the linked websites, or the information, products or services contained therein. For other than authorized VA activities, the Department does not exercise any editorial control over the information you may find at these locations. All links are provided with the intent of meeting the mission of the Department and the VA website. Please let us know about existing external links which you believe are inappropriate and about specific additional external links which you believe ought to be included.
Disclaimer of Liability: With respect to documents available from this server, neither the United States Government nor any of its employees, makes any warranty, express or implied, including the warranties of merchantability and fitness for a particular purpose, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights.
Reference from this web page or from any of the information services sponsored by the VA to any non-governmental entity, product, service or information does not constitute an endorsement or recommendation by the VA or any of its employees. We are not responsible for the content of any "off-site" web pages referenced from this server.
Disclaimer: The sharing of any non-VA information does not constitute an endorsement of products or services on the part of the VA.
![]()
VA Regional Offices
 The Veterans Benefits Administration (VBA) helps service members transition out of military service, and assists with Veterans with education, home loans, life insurance and much more. Service members, Veterans, their families, and Survivors are invited to request information on VA Benefits including disability compensation, pension, fiduciary, education, Veteran Readiness and Employment (VR&E), Home Loans, and Insurance. In addition to information on VA Benefits Veterans may initiate an intent to file and request assistance with filing compensation and pension claims. Visit regional office websites to learn about the services the regional office provides, directions to the facility, hours of operation, and the leadership team that serves the regional office.
The Veterans Benefits Administration (VBA) helps service members transition out of military service, and assists with Veterans with education, home loans, life insurance and much more. Service members, Veterans, their families, and Survivors are invited to request information on VA Benefits including disability compensation, pension, fiduciary, education, Veteran Readiness and Employment (VR&E), Home Loans, and Insurance. In addition to information on VA Benefits Veterans may initiate an intent to file and request assistance with filing compensation and pension claims. Visit regional office websites to learn about the services the regional office provides, directions to the facility, hours of operation, and the leadership team that serves the regional office.
Find out if you can get VA health care as a Veteran
The following four categories of Veterans are not required to enroll but are urged to do so to permit better planning of health resources:
- Veterans with a service-connected (SC) disability rated at 50% or more.
- Veterans seeking care for a disability the military determined was incurred or aggravated in the line of duty, but which VA has not yet rated, within 12 months of discharge.
- Veterans seeking care for a SC disability only or under a special treatment authority.
- Veterans seeking health registry examinations. VA’s health registry evaluation is a free, voluntary medical assessment for Veterans who may have been exposed to certain environmental hazards during military service. The evaluations alert Veterans to possible long-term health problems that may be related to exposure to specific environmental hazards during their military service. VA has established several health registries to track and monitor the health of specific groups of Veterans. You may be eligible to participate in one or more of these health registries: Agent Orange Registry, Airborne Hazards and Open Burn Pit Registry, Gulf War Registry (includes Operations Iraqi Freedom and New Dawn), Ionizing Radiation Registry, Depleted Uranium Follow-Up Program, and Toxic Embedded Fragment Surveillance Center. Use the chart below to help determine your eligibility.
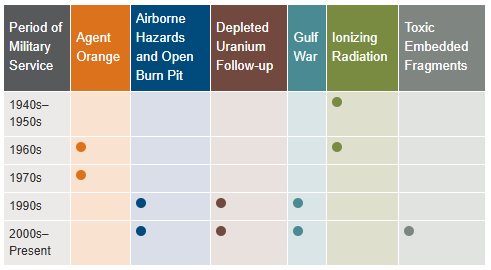
Find out how to apply for VA health care benefits as a Veteran or service member. For other mental health services, contact a VA medical center for information on eligibility and treatment options.
Community Care (Mission Act)
The MISSION Act became law in 2018, bringing the VA’s previous Veterans Choice Program to an end and establishing the Community Care Program. VA provides health care for Veterans from providers in your local community outside of VA. Veterans may be eligible to receive care from a community provider when VA cannot provide the care needed. This care is provided on behalf of and paid for by VA. Community care is also available to Veterans based on certain conditions and eligibility requirements, and in consideration of a Veteran’s specific needs and circumstances. VA offers urgent care services to eligible Veterans at in-network urgent care clinics to treat minor injuries and illnesses that are not life-threatening, such as colds, strep throat, sprained muscles, and skin and ear infections. Community care must be first authorized by VA before a Veteran can receive care from a community provider.
Vet Centers in VISN 20
 Vet Centers in VISN 20 are community-based counseling centers that provide a wide range of social and psychological services, including professional readjustment counseling to eligible Veterans, active-duty Army, Navy, Marine Corp, Air Force, Space Force, and Coast Guard service members, including National Guard and Reserve components, and their families. 1-877-927-8387 is an around the clock confidential call center where combat Veterans and their families can call to talk about their military experience or any other issue they are facing in their readjustment to civilian life. The staff is comprised of combat Veterans from several eras as well as families members of combat Veterans. This benefit is prepaid through the Veteran’s military service.
Vet Centers in VISN 20 are community-based counseling centers that provide a wide range of social and psychological services, including professional readjustment counseling to eligible Veterans, active-duty Army, Navy, Marine Corp, Air Force, Space Force, and Coast Guard service members, including National Guard and Reserve components, and their families. 1-877-927-8387 is an around the clock confidential call center where combat Veterans and their families can call to talk about their military experience or any other issue they are facing in their readjustment to civilian life. The staff is comprised of combat Veterans from several eras as well as families members of combat Veterans. This benefit is prepaid through the Veteran’s military service.
Alaska
| Anchorage Vet Center (Anchorage, AK) | Fairbanks Vet Center (Fairbanks, AK) |
| Kenai Vet Center Outstation (Soldotna, AK) | Wasilla Vet Center (Wasilla, AK) |
Idaho
| Boise Vet Center (Boise, ID) | East Idaho Vet Center (Idaho Falls, ID) |
Oregon
| Central Oregon Vet Center (Bend, OR) | Eugene Vet Center (Eugene, OR) |
| Grants Pass Vet Center (Grants Pass, OR) | Portland, OR Vet Center (Portland, OR) |
| Salem Vet Center (Salem, OR) |
Washington
Plan your trip to VA
 In 1946, Veterans Canteen Service (VCS) was established by law to provide comfort and well-being to America’s Veterans. With our many retail stores, cafés and coffee shops across the country, we serve those who have served our country. We are a self-sustaining entity providing merchandise and services to Veterans enrolled in VA’s healthcare system, their families, caregivers, VA employees, volunteers and visitors. We are honored to give back to the VA community through many programs established for the health and well-being of our nation’s heroes. Revenues generated from VCS are used to support a variety of programs, such as VA’s Rehabilitation Games, Fisher Houses, Poly-Trauma Centers for OIF/OEF/OND Veterans, disaster relief efforts, Substance Abuse Cessation, VA’s Homelessness initiatives, Women Veterans, Veteran Suicide Prevention and other activities.
In 1946, Veterans Canteen Service (VCS) was established by law to provide comfort and well-being to America’s Veterans. With our many retail stores, cafés and coffee shops across the country, we serve those who have served our country. We are a self-sustaining entity providing merchandise and services to Veterans enrolled in VA’s healthcare system, their families, caregivers, VA employees, volunteers and visitors. We are honored to give back to the VA community through many programs established for the health and well-being of our nation’s heroes. Revenues generated from VCS are used to support a variety of programs, such as VA’s Rehabilitation Games, Fisher Houses, Poly-Trauma Centers for OIF/OEF/OND Veterans, disaster relief efforts, Substance Abuse Cessation, VA’s Homelessness initiatives, Women Veterans, Veteran Suicide Prevention and other activities.
VCS operates over 200 Patriot Stores in Veterans Administration (VA) Medical Centers nationwide. Many of our stores have been recently updated and expanded to provide our customers with a modern, clean and comfortable shopping experience. Our stores welcome our customers with wider aisles, wood-like floors, enhanced lighting and directional signage. PatriotStores have expanded hours of operation to provide service for customers on weekends at most locations.
The Patriot Cafe is the best place in the VA Medical Center to enjoy delicious, freshly prepared breakfast or lunch served hot or cold each weekday. Providing Veterans, their families, VA employees, volunteers and visitors a place to relax and enjoy a meal or take-out for their convenience. With a wide variety of food from traditional comfort food, specialized menu selections and a large assortment of healthy choices; there is something for everyone's taste buds.
Hospital Service Directory
To find out whether there is a van near you use the Disabled American Veterans (DAV) Hospital Service Coordinator Directory to contact your nearest HSC for information or assistance. Please remember that the DAV Transportation Network is staffed by volunteers; therefore, it is unable to cover every community. The vans are driven by volunteers, and the rides coordinated by more than 133 Hospital Service Coordinators around the country. Our nation’s heroes travel around the globe to protect our freedoms—it’s only right that we return their dedication. Volunteering to drive a Vet ensures that even those living remotely from VA hospitals can make their appointments and never go without the treatment they need. Learn more about the DAV transportation network through the VISN 20 NewsFlash Resources section.
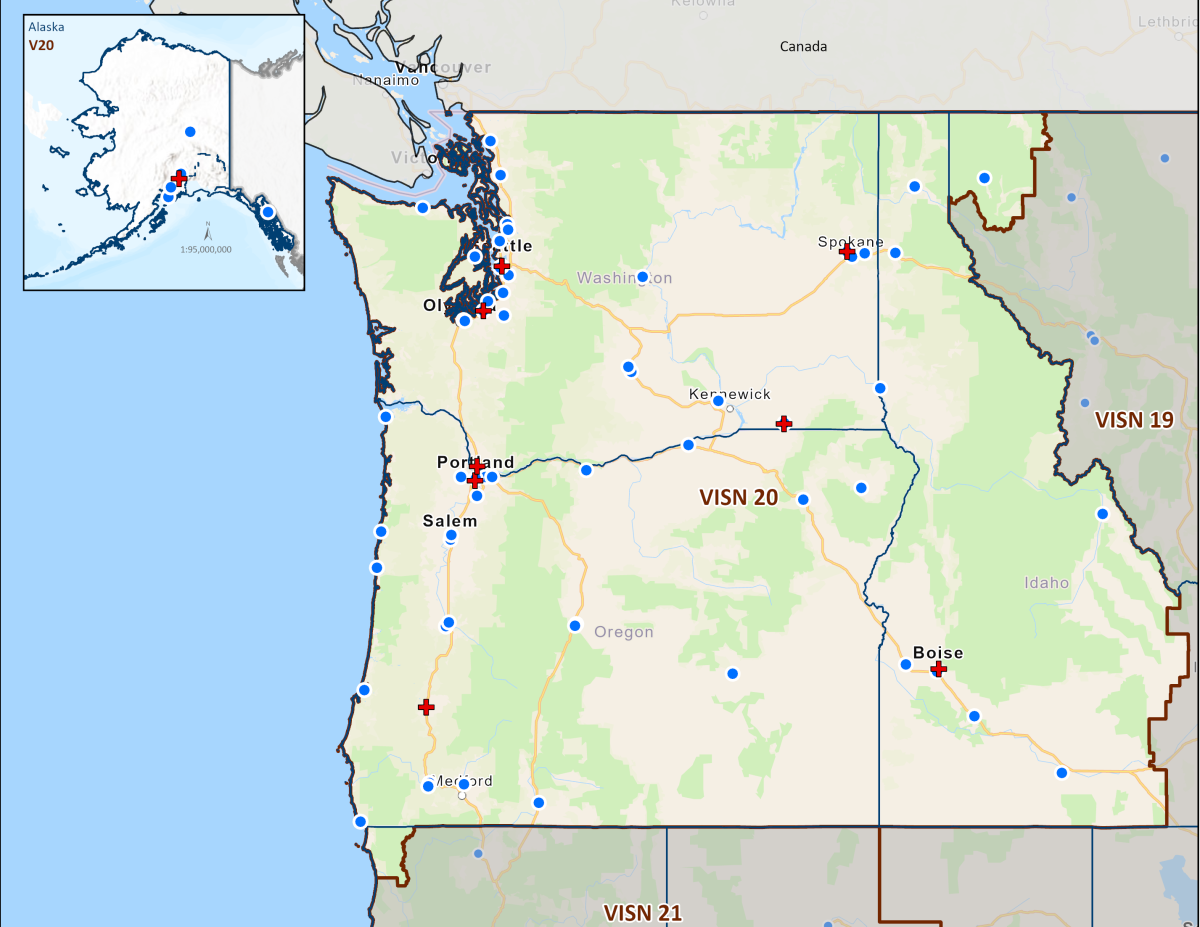 Today's VHA - the largest of the three administrations that comprise the VA - continues to meet Veterans' changing medical, surgical, and quality-of-life needs. VHA is the largest integrated health care system in the United States, providing care at 1,321 health care facilities, including 172 VA Medical Centers and 1,138 outpatient sites of care of varying complexity (VHA outpatient clinics) to over 9 million Veterans enrolled in the VA health care program. There are 18 Veterans Integrated Service Networks (VISNs) in VHA operating as regional systems of care to better meet local health care needs and provides greater access to care. In the Pacific Northwest, VISN 20 serves Veterans in Alaska, Oregon, Washington, most of Idaho, and one county each in California and Montana. Spanning 23% of the US land mass, VISN 20 is the largest geographic region of VA. Operating across three time zones over 817,417 square miles, VISN 20 is home to 273 federally recognized American Indian and Alaskan Native tribes. According to DoD, American Indians and Alaska Natives have one of the highest representations in the United States Armed Forces. VA consults with American Indian and Alaska Native tribal governments to develop partnerships that enhance access to services and benefits by Veterans and their families. VA is committed to ensuring that Native American Veterans and their families are able to utilize all benefits and services they are entitled to receive. As of the end of FY2024, 39% of VISN 20 enrollees resided in rural or highly rural areas.
Today's VHA - the largest of the three administrations that comprise the VA - continues to meet Veterans' changing medical, surgical, and quality-of-life needs. VHA is the largest integrated health care system in the United States, providing care at 1,321 health care facilities, including 172 VA Medical Centers and 1,138 outpatient sites of care of varying complexity (VHA outpatient clinics) to over 9 million Veterans enrolled in the VA health care program. There are 18 Veterans Integrated Service Networks (VISNs) in VHA operating as regional systems of care to better meet local health care needs and provides greater access to care. In the Pacific Northwest, VISN 20 serves Veterans in Alaska, Oregon, Washington, most of Idaho, and one county each in California and Montana. Spanning 23% of the US land mass, VISN 20 is the largest geographic region of VA. Operating across three time zones over 817,417 square miles, VISN 20 is home to 273 federally recognized American Indian and Alaskan Native tribes. According to DoD, American Indians and Alaska Natives have one of the highest representations in the United States Armed Forces. VA consults with American Indian and Alaska Native tribal governments to develop partnerships that enhance access to services and benefits by Veterans and their families. VA is committed to ensuring that Native American Veterans and their families are able to utilize all benefits and services they are entitled to receive. As of the end of FY2024, 39% of VISN 20 enrollees resided in rural or highly rural areas.
VA Medical Centers within VISN 20
Colonel Mary Louise Rasmuson Campus of the Alaska VA Healthcare System, Anchorage, Alaska
VA Boise Medical Center of the Boise VA Healthcare System, Boise, Idaho
VA Portland Medical Center of the Portland VA Healthcare System, Portland, Oregon
VA Roseburg Medical Center of the Roseburg VA Healthcare System, Roseburg, Oregon
VA White City Medical Center of the VA Southern Oregon Healthcare System, White City, Oregon
VA Seattle Medical Center of the VA Puget Sound Healthcare System, Seattle, Washington
Mann-Grandstaff Department of Veterans Affairs Medical Center, Spokane, Washington
Jonathan M. Wainwright Memorial VA Medical Center of the VA Walla Walla Healthcare System, Walla Walla, Washington
VA Puget Sound Health Care System (VAPSHCS) serves Veterans from a five-state area in the Pacific Northwest with two main divisions: American Lake VA Medical Center and Seattle VA Medical Center. Veterans Medical Centers are also located in Spokane, Vancouver, and Walla Walla. VA Outpatient Clinics and Vet Centers are located in Bellingham, Bellevue, Bremerton, Edmunds, Everett, Federal Way, Lacey, Mount Vernon, Olympia, Port Angeles, Puyallup, Richland, Renton, Silverdale, Seattle, Spokane, Union Gap, Vancouver, Walla Walla, Wenatchee, and Yakima.
VA Portland Health Care System (VAPORHCS) serves Veterans in Oregon and Southwest Washington with two main divisions: Portland VA Medical Center and Vancouver VA Medical Center. Veterans Medical Centers are also located in Roseburg, White City, and Vancouver, Washington. VA Outpatient Clinics and Vet Centers are located in Astoria, Bend, Boardman, Brookings, Eugene, Fairview, Grants Pass, Hines, Hillsboro, Klamath Falls, LaGrande, Lincoln City, Newport, Portland, Salem, The Dalles, and West Linn.
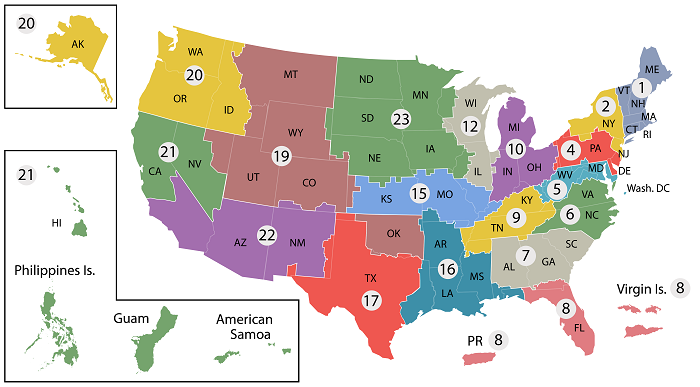
- VISN 1: VA New England Healthcare System
- VISN 2: New York/New Jersey VA Health Care Network
- VISN 4: VA Healthcare - VISN 4
- VISN 5: VA Capitol Health Care Network
- VISN 6: VA Mid-Atlantic Health Care Network
- VISN 7: VA Southeast Network
- VISN 8: VA Sunshine Healthcare Network
- VISN 9: VA MidSouth Healthcare Network
- VISN 10: VA Healthcare System
- VISN 12: VA Great Lakes Health Care System
- VISN 15: VA Heartland Network
- VISN 16: South Central VA Health Care Network
- VISN 17: VA Heart of Texas Health Care Network
- VISN 19: Rocky Mountain Network
- VISN 20: Northwest Network
- VISN 21: Sierra Pacific Network
- VISN 22: Desert Pacific Healthcare Network
- VISN 23: VA Midwest Health Care Network



















